Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Professor Soo Hyun Eom is the first to identify structure and mechanism of protein structure that regulates cell survival
- 엘리스 리
- REG_DATE : 2017.01.16
- HIT : 930
Professor Soo Hyun Eom is the first to identify structure
and mechanism of protein structure that regulates cell survival

(From left) Professor Soo Hyun Eom, Ph.D. student Kyoung Ryoung Park, and Dr. Min Sung Kwon
Within the 40 trillion or so cells of the human body, the structure and mechanism of the protein (EFhd2 *) have been shown to be critical components of cell survival and regulation, such as cell shape preservation, migration, and cell division.
EFhd2: A protein that binds to the cytoskeletal protein (actin), an essential element of cell migration and cleavage. The actin binding site of EFhd2 contains an EF-hand domain essential for calcium binding.
□ The National Research Foundation of Korea (Director Sajang Lee) stated that "Professor Soo Hyun Eom of the Gwangju Institute of Science and Technology has, for the first time, revealed the three-dimensional structure of EFhd2, which a novel cytoskeleton-regulating protein *, in high-resolution and also revealed the mechanism of function."
* New cytoskeleton regulatory protein: a protein that interacts with the cytoskeleton. Controls the phenomena essential for cell survival, such as cell shape maintenance, migration, and cell division.
□ EFhd2 is expressed extensively in the cell proximal portion of the cell. Overexpression of EFhd2 results in an increase in cell migration rate, thereby affecting cancer cell metastasis. In Alzheimer"s patients, it coagulates with tau * mutants in brain cells and acts as a pathogen. To date, the three-dimensional structure and functional mechanism of EFhd2 protein has not been elucidated.
* Tau: a protein that stabilizes the microtubules in the cell and causes Alzheimer"s disease
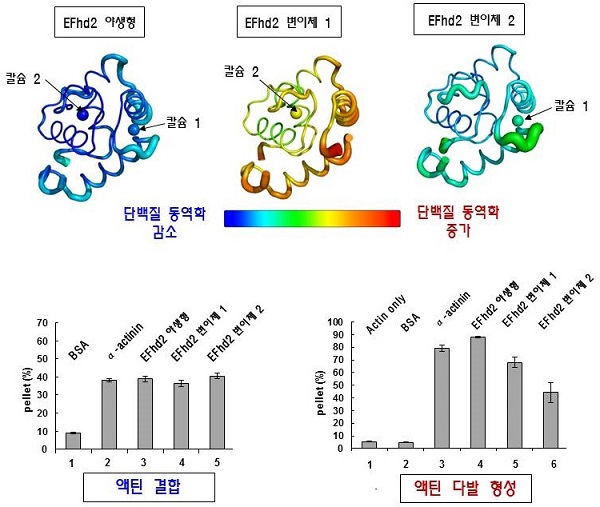
(Figure 1) Comparison of structural dynamics between wild type and mutant of human EFhd2. Three dimensional structure of the human EFhd2 actin binding site. The actin binding site of wild-type EFhd2 binds two calcium molecules. If it does not bind calcium (mutant 1, variant 2), the structural dynamics are increased. Changes in structural dynamics due to calcium binding directly affect the actin bundling function of EFhd2.
Using an X-ray crystallography technique, the team uncovered a three-dimensional structure that did not bind calcium-bound structures to EFhd2 in our bodies. The actin * binding site of EFhd2 contains the EF-hand * domain, which is essential for calcium binding. When the calcium binds to this, the structure of the actin * binding site is stabilized, and the actin bundle, inducing function, becomes possible. On the other hand, when calcium is not bound to the EF-hand domain, the actin binding site is changed into a dynamic structure. These structural changes were found to be the cause of the decrease of actin bundle formation function of calcium-free EFhd2. This means that the cytoskeletal regulation by EFhd2 is due to the structural dynamics of calcium binding.
* EF-hand: A form with two alpha helices connected by a loop. The basic structure that appears mainly in calcium binding proteins
* X-ray crystallography: Obtain an X-ray diffraction pattern from crystals of biomolecules and obtain an electron density map to analyze the high-resolution three-dimensional structure at the atomic level
Actin: A fiber protein necessary for cytoskeletal retention. Actin filaments are made of polymers. Several strands are gathered to form actin bundles.
□ Professor Soo Hyun Eom said, "This research was the first to reveal how the mechanism of actin bundle regulation depends upon on calcium binding of EFhd2, which is involved in cell survival regulation. We are expecting to contribute to the development of anticancer drugs aimed at inhibiting cancer metastasis, and to develop various treatments for neurodegenerative diseases, * including Alzheimer"s disease and dementia."
* Neurodegenerative disease: A concept that collectively refers to diseases caused by gradual neuronal death in certain parts of the nervous system
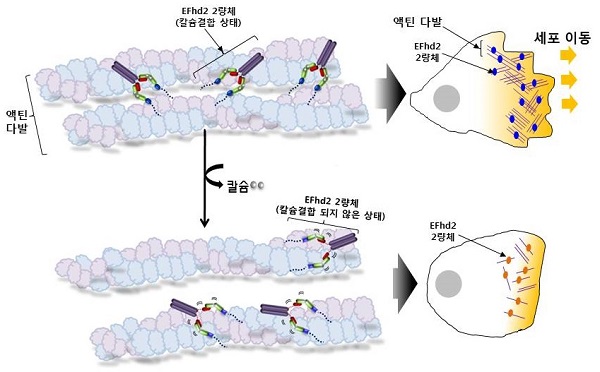
(Figure 2) Schematic representation of actin bundle formation mechanism of human EFhd2. EFhd2 forms an actin bundle in a calcium-bonded dimeric form. If calcium is not bound, it will directly affect the structural dynamics of the two actin binding sites in the dimeric state, resulting in reduced actin bundle formation. (* Dimer: a form in which two molecules are gathered to show activity, EFhd2 is a dimer as a basic unit, and the actin bundle forming function is activated).
□ The research was supported by the Ministry of Science, ICT and Future Planning (Individual Research), the Biomedical Technology Development Project, and the Radiation Technology Development Project and was published on December 15, 2016, in Scientific Reports.