Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Research team led by GIST Professor Soo Hyun Eom discovers structural properties of human quinolinate phosphoribosyltransferase that may lead to new treatment option for malignant glioma
- 엘리스 리
- REG_DATE : 2016.03.18
- HIT : 1200
Research team led by GIST Professor Soo Hyun Eom discovers structural properties of human quinolinate phosphoribosyltransferase that may lead to new treatment option for malignant glioma
Research findings published in Scientific Reports
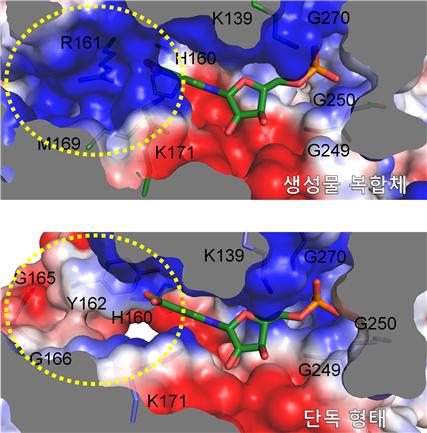
Structural comparison of substrate binding sites. Surface representations of substrate binding pockets between HsQPRT-NAMN (green) and HsQPRT-open (blue). Positive and negative surface charges of the protein are colored in blue and red, respectively. The NAMN molecule is shown as sticks.
Quinolinate phosphoribosyltransferase (QPRT) catalyses the production of nicotinic acid mononucleotide, a precursor of de novo biosynthesis of the ubiquitous coenzyme nicotinamide adenine dinucleotide. QPRT is also essential for maintaining the homeostasis of quinolinic acid in the brain, a possible neurotoxin causing various neurodegenerative diseases. Although QPRT has been extensively analysed, the molecular basis of the reaction catalysed by human QPRT is not well understood.
Therefore, researchers at the Gwangju Institute of Science and Technology (GIST) led by Professor Soo Hyun Eom of the School of Life Sciences and the Steitz Center for Structural Biology, have found that the interaction between dimeric subunits was dramatically altered during the reaction process by conformational changes of two flexible loops in the active site at the dimer-dimer interface. In addition, the N-terminal short helix α1 was identified as a critical hexamer stabilizer. The structural features, size distribution, heat aggregation and ITC studies of the full-length enzyme and the enzyme lacking helix α1 strongly suggest that human QPRT acts as a hexamer for cooperative reactant binding via three dimeric subunits and maintaining stability. Based on their comparison of human QPRT structures in the apo and complex forms, the researchers propose a drug design strategy targeting malignant glioma.

(From left to right) Professor Soo Hyun Eom, Ph.D. candidtate Hyung-Seop Youn, and Dr. Tae Gyun Kim
The findings of their research was published by Scientific Reports in a paper entitled "Structural Insights into the Quaternary Catalytic Mechanism of Hexameric Human Quinolinate Phosphoribosyltransferase, a Key Enzyme in de novo NAD Biosynthesis" that was authored by Hyung-Seop Youn, Tae Gyun Kim, Mun-Kyoung Kim, Gil Bu Kang, Jung Youn Kang, Jung-Gyu Lee, Jun Yop An, Kyoung Ryoung Park, Young jin Lee, Young Jun Im, Jun Hyuck Lee, and Soo Hyun Eom.
This research was supported by grants obtained from the National Research Foundation funded by the Korea government, the Basic Science Research Program, ICT & Future Planning, the “BK21 Plus Program” funded by the Ministry of Education, Korea Healthcare Technology R&D Project, “Systems biology infrastructure establishment,” and the “Steitz Center for Structural Biology” grant provided by the Gwangju Institute of Science and Technology.
GIST Professor Soo Hyun Eom explained the significance of the research findings by saying, "This study provides the first insights into how QPTR regulates the level of quinolinic acid within the brain, which is a possible neurotoxin that may contribute to several neurodegenerative diseases, such as Alzheimer"s, epilepsy, and may even cause brain tumors. By providing a better understanding of this process, new therapeutic treatment options can be developed to fight these diseases."