Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] GIST Professors Soo Hyun Eom and Do Han Kim identify proteins regulating mitochondrial calcium uptake
- 엘리스 리
- REG_DATE : 2015.10.14
- HIT : 863
Proteins regulating mitochondrial calcium uptake identified
- Provides important clues for understanding cardiovascular
and nervous system diseases

(From left) GIST Professor of Biological Sciences Soo Hyun Eom, Professor Do Han Kim,
Ph.D. candidate Youngjin Lee, and Dr. Choon Kee Min
□ Creation of Future Science (Minister Yang Hui Choi) and the Korea Research Foundation (Chairman Jung Min Guen) have announced that a Korean research team has just discovered the pathway role of calcium in human mitochondrial proteins ** * by being the first in the world to identify high-resolution, three-dimensional structures and function of the "mitochondrial calcium uniporter."
* Mitochondria: cell organelles that contribute to respiratory and energy synthesis and regulates calcium in the cell
** Membrane protein: proteins bound to the membrane of the cells or organelles
□ Gwangju Institute of Science and Technology professor Soo Hyun Eom and professor Do Han Kim professors performed the research with the support of the Steitz Center for Structural Biology at the GIST Nobel Research Institute as well as support from the Korea Research Foundation and the Future Creation Science. Their research was published--and selected as the issue"s cover--in the prestigious journal of EMBO Reports Biology (EMBO Reports) on October 1, 2015.
○ The paper"s title and author information are as follows:
- Title: Structure and function of the N-terminal domain of the human mitochondrial calcium uniporter
- About the authors: Professor Soo Hyun Eom, Professor Do Han Kim (co-corresponding author, Gwangju Institute of Science and Technology) Youngjin Lee Ph.D. candidatte, and Dr. Choon Kee Min (co-first author, Gwangju Institute of Science and Technology)
□ The main content of the paper is organized as follows:
1. Research Motivation
○ A cell"s mitochondria are essential organelles for performing various functions, such as the energy synthesis and survival signaling by regulating the intracellular concentration of calcium. However, a coherent understanding of the mechanisms for calcium uptake was not clear until now.
○ In 2011, it was reported for the first time in the academic world that mitochondria endometrial membrane proteins of mitochondrial calcium uniporter has a pathway role for calcium uptake. Abnormalities in this mechanism has been known to cause various diseases as well as death. However, the three-dimensional structure of the mitochondrial calcium uniporter was not known until now.
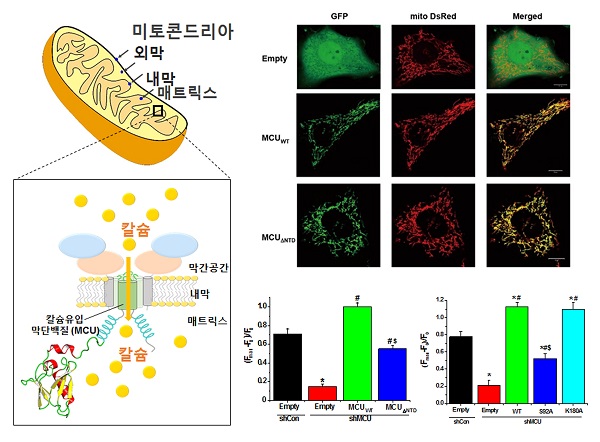
Figure 1. Mechanism of adjustment on the N- end of the mitochondrial calcium calcium uptake uniporter
The mitochondrial outer membrane is composed of intervening space and the lining matrix. Uni mitochondrial calcium in the mitochondria inner Porter act as a calcium uptake pathway, and through the inner mitochondrial perform functions that calcium uptake into the control (left). Mitochondrial calcium uniporter N- terminal part is deleted, revealing that mutations to the serine 92 amino acid to the alanine amino acid significantly inhibits the uptake of calcium in the mitochondria of human cells (right).
2. Methodology
○ Human mitochondrial calcium uniporter complex were ascertained by passing the crystalline protein structure through an X-ray beam analysis, and high-resolution, three-dimensional structures were identified through computational modeling.
* N-terminal domain: A protein is made by starting from the N-terminal to the C- terminal end of a polypeptide chain.
○ Confirmation of calcium uptake inhibition can be made by removing the N- terminal portion or by making a mutation to the amino acids (serine 92) that are expected to affect calcium uptake in the N- terminal domain and by adding a fluorescing material to the mutant protein to detect any momentary changes of the incoming calcium into the mitochondria.
3. Results
○ High-resolution three-dimensional structures of the human mitochondrial calcium uniporter complex of the N- terminal were revealed for the first time in the world, and that confirmed a new type of protein folding* that has not yet been reported.
* Protein folding: A peptide chain of the protein molecule is folded into a unique shape to form a stable subunit structure.
○ The mitochondrial calcium uniporter N- terminal domain reveals the importance of mitochondria in the regulation of calcium uptake suggests that it may play a key role in mitochondrial function control through interaction with various proteins.
Articles cover

Figure 2. The tertiary protein structure of the mitochondrial calcium uniporter N- terminal region
□ · Professors Soo Hyun Eom and Do Han Kim who participated in the research stated the significance of the study by saying, "Cell death caused by excessive mitochondrial calcium uptake can cause cardiovascular disease, neurological disorders, such as increased myocardial infarction*, epilepsy**, and brain metastases. By focusing on the mitochondrial calcium uniporter in the present study, it is expected to provide a significant insights into the development of new therapies for diseases."
* Myocardial infarction (myocardial infarction): If blood vessels in the heart are blocked due to acute thrombosis or the sudden constriction of blood vessels, reduced oxygen and nutrient supplies to the whole or part of the heart will cause the muscles and cells of the heart to die.
** Epilepsy: A physical abnormality that can cause seizures.