Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Development of new technology to treat gout 7 times more effectively
- 엘리스 리
- REG_DATE : 2015.05.28
- HIT : 771
A team led by Prof. Inchan Kwon developed a treatment for gout that is 7 times more effective than current methods
Professor Inchan Kwon (left) and doctoral candidate Sung-In Lim of the University of Virginia, USA
Korean researchers have developed a new technology to keep urate oxidase longer inside the body to break. Urate oxidase breaks down the uric acid that causes gout and converts it into harmless substance. In the future, this treatment is expected to significantly shorten treatment for gout and reduce the suffering of patients.
* Uric acid is a waste product that results from the body"s normal process of cells dying and releasing chemicals, called purines.
* Urate oxidase is an enzyme belonging to the purine degradation pathway, whose function is to prevent build-up of uric acid by catalyzing the oxidation of uric acid;
GIST professor Inchan Kwon lead the research and doctoral candidate Sung-In Lim of the University of Virginia, USA, conductedthe research and was supported by the ‘Basic Research Projects’ of the Future Creation Sciences and the National Research Foundation of Korea. Their paper was published on April 15, 2015, in the ‘Journal of Controlled Release’ and the team completed its research result for a domestic patent application.
Uric acid in the blood is the main cause of gout. Gout is an incurable inflammatory diseases that is caused by uric acid which damages the kidneys or crystalizes in cartilage and tendons. The number of patients with gout has increased by an annual average of 13% over the past five years in Korea. Many studies have been conducted to cure gout by controlling the amount of uric acid, but those research could not retain the efficacy of the urate oxidase.
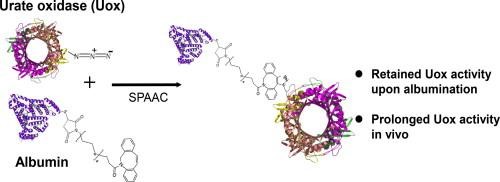
Process of albumin fusion to urate oxidase through Strain-promoted Azide-Alkyne Cycloaddition
Albumin fusion/conjugation (albumination) has been an effective method to prolong in vivo half-life of therapeutic proteins. However, its broader application to proteins with complex folding pathway or multi-subunit is restricted by incorrect folding, poor expression, heterogeneity, and loss of native activity of the proteins linked to albumin.
Site-specific albumination enabled by non-natural amino acid incorporation and orthogonal chemistry demonstrates its promise for the development of long-acting protein therapeutics with high potency and safety.
“The research will contribute significantly to increase the efficacy of the drugs to treat excessive uric acid, such as for gout and other diseases,” Prof. Inchan Kwon said, “and is expected to be applied to improving thelong lasting efficacy of other protein based drugs, such as interferon."