Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
Research team led by Professor Jaeyoung Lee develops develops high-efficiency non-platinum catalyst by removing carbon layers
- 김슬혜
- REG_DATE : 2014.06.11
- HIT : 1080
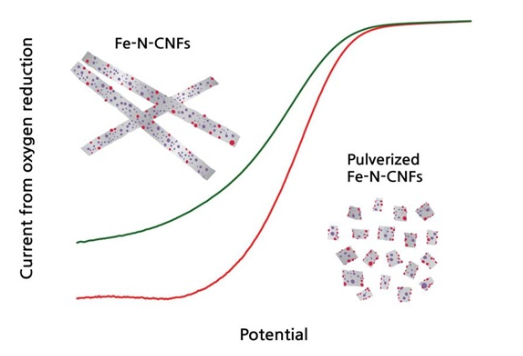
Research team led by Professor Jaeyoung Lee develops high-efficiency non-platinum catalyst by removing carbon layers - uses ceramic beads to raise efficiency to 70%↑…research finding published by ChemSusChem - expected to help commercialization of lithium air battery for smart grid and electric vehicles □ A group of Korean researchers developed a new non- platinum catalyst to be used in the lithium-air battery** touted as one of post batteries* by using a simple mechanical grinding method. This new catalyst can replace expensive platinum catalyst which is considered the most effective catalyst available today and enables highly-efficientoxygen-reduction reaction. Therefore, it is expected to advance the commercialization of the lithium-air battery to be used in smart grid and electric vehicles. *Post batteries were singled out by Samsung Economic Research Institute as one of seven most dramatic innovation technologies to change industries in the future. Post batteries have a significantly higher energy capacity than the lithium ion battery. **The lithium-air battery raises energy density per unit of weight : The lithium air battery changed the anode of lithium transition metal oxide into light cathode and useslithium-metal electrode instead of graphite electrode to storelithium ions between layers, thereby significantly increasing energy density per unit of weight. However, its downside is lower power output compared with lithium ion batteries due to difficulty in maintaining stable capacity for charging/discharging and lower reaction speed of oxygen reducing to lithium oxide. ○ This research was led by Professor Jaeyoung Lee of School of Environmental Science and Engineering of GIST (Gwangju Institute of Science and Technology, President ž Young Joon Kim) (corresponding author, Ertl Catalyst Research Institute) and carried out by Mr. Cheong Bum Gyun in the doctoral program at School of Environmental Science and Engineering (leading author). It was sponsored by the energy storage research foundation-building project of Research Institute for Solar and Sustainable Energies (RISE) of GIST and the international joint technology development project of Korea Institute for Advancement of Technology (KIAT), and the research finding was published by ChemSusChem, a world-class energy & chemical journal, in May. * Title: Excavated Fe-N-C sites for enhanced electrolyitic activity in oxygen reduction reaction (Volume 7, Issue 5, pages 1289–1294, May 2014 / DOI: 10.1002/cssc.201301374) □ The most urgent problem to be addressed in the development of the lithium-air battery, which is touted as a promising energy conversion & storage device to succeed the lithium-ion battery used in cell phones, is how to increase the speed of oxygen reduction reaction*. *Oxygen reduction reaction (ORR) refers to a reaction that oxygen is reduced to water. This is an essential part of the process where chemical energy is converted into electric energy. In order to commercialize post batteries and improve their performances, the efficiency of oxygen reduction reaction should be raised to the highest possible extent. ○ Today, platinum is the most effective catalyst to facilitate oxygen reduction reaction but is too expensive. In order to promote the use of the lithium-air battery, it is very important to develop a non-platinum catalyst to replace platinum. (picture 1) Catalyst activation was increased by exposing iron molecules which are contained within the carbon structure of carbon nanofibers onto its surface by mechanical grinding method (ball-mill process) (red graph) Lower the graph of ground AFe-N-CNFs, the more oxygen reduction. (green graph) Fe-N-CNFs before grinding. □ The research team manufactured 0.1㎛ carbon fibers consisting of Fe-Nx-C by electric radiation* and high-temperature heat treatment. In order to improve catalyst performance for oxygen reduction, the fibers were put into a grinder together with 2㎜ zirconium oxide beads so as to remove carbon layers. *Electric radiation is a method to manufacture ultra-fine fibers (less than 1㎛) by lengthening viscous polymer solution by high voltage. ○ When iron-carbon fibers whose carbon layers were removed by high speed mechanical grinding were used as a catalyst, the efficiency of oxygen reduction reaction improved to 73% of that of platinum catalyst. It was 1.7 times higher than the efficiency of existing non-platinum catalysts. □ Professor Lee said “We discovered the fact that catalysts to reduce oxygen are mostly located around metal particles, which provides a clue to understanding the process of how iron-nitrogen-carbon catalysts form. We expect that this research finding will contribute to the commercialization of mid-to-large energy storage systems linked with electric vehicles and renewable energy.”