Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press release] Professor Inchan Kwon leads research team to develop a new fluorescent labeling technique and identifies a new protein-membrane interaction principle
- 엘리스 리
- REG_DATE : 2016.09.20
- HIT : 983
Professor Inchan Kwon leads research team to develop a new fluorescent
labeling technique and identifies a new protein-membrane interaction principle

(From left to right): Professor Inchan Kwon, Professor Lukas K. Tamm, Dr. Sung In Lim, and Dr. Sung-Tae Yang
Fluorescent labeling allows researchers to detect and analyze complex interactions of various biomolecules by binding a fluorophore to a specific site on a target molecule, and this method has been widely used to help researchers understand the interactions between proteins and the cell membrane. However, various methods of florescent labeling can damage the biomolecule"s original function or interfere with protein folding or other cellular activity.
To overcome these limitations regarding fluorescent labeling of biomolecules, Professor Inchan Kwon, who is both a member of the School of Materials Science and Engineering at the Gwangju Institute of Science and Technology (GIST) and of the Department of Chemical Engineering at the University of Virginia (UVA), has led an international research team to develop a site-specific fluorescent labeling technique that minimizes the structural and functional changes to proteins.
Professor Kwon"s research team was able to do this by developing a site-specific labeling procedure that combines the genetic incorporation of a reactive non-natural amino acid known as p-azido-L-phenylalanine (AZF) with the application of bio-orthogonal chemistry, which allowed the researchers to measure the functional and Ca2+-dependent translocation of recoverin to membranes. The translocation of the recoverin, which is a calcium-binding protein from the retina, was shown to depend on the curvature of the target membrane as well as the lipid packing density in that membrane. Recoverin favors translocating to liquid-disordered regions of model membranes with coexisting Lo- and Ld-phase domains.
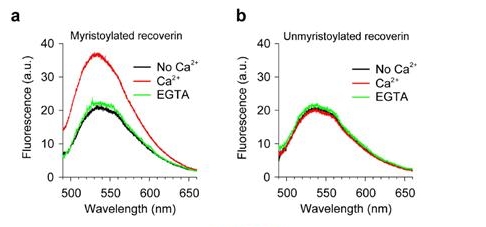
NBD-labeled myristoylated (mRec-F23NBD) and unmyristoylated (Rec-F23NBD) versions of recoverin were used for spectrometric measurements of membrane association. Fluorescence emission spectra of 0.1 μM mRec-F23NBD (a) and Rec-F23NBD (b) were recorded in LUVs (0.1 mM total lipids) composed of PC/PE (7:3 mol:mol) in the absence (black) and presence (red) of 1 mM Ca2+. 2 mM EGTA was added after 1 h incubation of recoverin with 1 mM Ca2+ (green).
Because many proteins have similar limitations for fluorescent labeling as recoverin, the strategy proposed by Professor Kwon"s research team is very general and will be beneficial for examining a large group of protein-membrane interactions and should be helpful to answer many important questions of cell signaling in model and cell membranes that involve tightly controlled protein interactions on membranes.
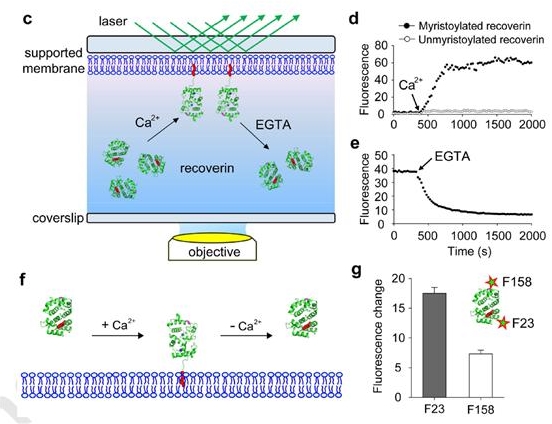
(c) Schematic of TIRF microscopy approach used to monitor the association and dissociation of recoverin to supported membranes. (d) Binding of 0.1 μM mRec-F23NBD and Rec-F23NBD to supported membranes composed of PC:PE (7:3 mol:mol). Mean fluorescence intensity over time, indicating recoverin binding to the membrane after addition of 1 mM Ca2+. (e) Dissociation of mRec-F23NBD from supported membranes. Mean fluorescence intensity over time, indicating recoverin dissociation from the membrane after addition of 2 mM EGTA. (f) Schematic diagram of recoverin-membrane interaction. The myristoyl group of recoverin is exposed by the Ca2+ binding and inserts into the membranes. Such recoverin binding can be dissociated from the membrane by Ca2+removal. (g) Position-dependent NBD fluorescence intensity of membrane-bound recoverin.
Their paper entitled "Site-specific fluorescent labeling to visualize membrane translocation of a myristoyl switch protein" was authored by Sung-Tae Yang, Sung In Lim, Volker Kiessling, Inchan Kwon, and Lukas K. Tamm and was published on September 8, 2016, by Scientific Reports.
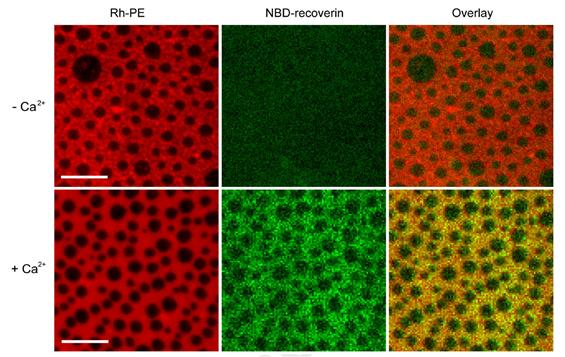
Supported membranes with coexisting Lo/Ld phases (left panels) were composed of DPPC:DOPC:Cholesterol (2:2:1). The membranes were labeled with 0.1 mol% Rh-PE, which preferentially partitions into the Ld phase. Myristoyl-Rec-F23NBD was added to the membranes in the absence (top row) and presence (bottom row) of 1 mM Ca2+ (middle panels). The overlay (right panels) shows that mRec-F23NBD (green) associates with the Ld phase (red) on supported membranes in the presence of Ca2+. Scale bars are 10 μm. Representative images of three experiments.
Professor Inchan Kwon explained the significance of the research by saying, "At this time, the development of our florescent labeling technique will help researchers better understand the signaling pathways of protein-membrane interaction as well as other biomolecules. This in turn will help us better understand the causes of various diseases, which will contribute to the development of new drugs."