Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
Energy metabolism in microbial strains can convert waste gas into fuel or bio compounds
- 엘리스 리
- REG_DATE : 2015.07.10
- HIT : 879
Energy metabolism in microbial strains can
convert waste gas into fuel or bio compounds


Professor In Seop Chang of the School of Environmental Science and Engineering and Ph.D. candidate Ji-yeong Jeong collaborated with German researchers to identify microbes whose energy metabolism can convert waste gas (syngas) into bio raw materials or biofuel.
The research was supported by the New and Renewable Energy Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) in collaboration with Professor Volker Müller of the Molecular Biosciences department of Goethe University Frankfurt and Ph.D. candidate Johannes Bertsch.
The research was selected as the ‘Spotlight Article’ and published in the online publication of Applied and Environmental Microbiology on May 8, 2015.
Eubacterium limosum KIST612 grows on syngas. It is one of the few acetogens known to produce butyrate and may become an interesting alternative in the production of higher carbon chain compounds from gases. A prerequisite for improving product yields is understanding the biosynthetic pathway and its energetics. To address this question, the researchers used a genome-guided experimental approach to identify the biosynthetic route for acetyl-CoA (coenzyme A) and butyrate and their coupling to energy conservation in E. limosum KIST612.
The conversion of gases to acetate is of special interest to researchers because it is a promising method of producing biofuels and biocommodities without reliance on carbohydrates. The conversion of syngas, a mixture of H2, CO, and CO2, to ethanol by microbial fermentation has already been demonstrated in large industrial-scale pilot plants by companies like Coskata, INEOS Bio, and LanzaTech.
Acetogenic bacteria are a phylogenetically diverse group of strictly anaerobic bacteria able to reduce two molecules of CO2 to acetate. Electrons may derive from molecular hydrogen (autotrophic growth), from carbon monoxide, or from organic donors (heterotrophic growth) such as hexoses, pentoses, formate, lactate, alcohols, or methyl group donors.
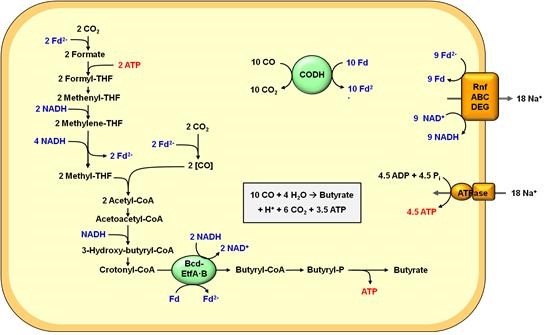
Energy conservation during CO-dependent butyrate formation by Eubacterium limosum KIST612. A ferredoxin-dependent formate dehydrogenase and an electron-bifurcating methylene-THF reductase are assumed. NADH is generated from reduced ferredoxin by action of the Rnf complex, which translocates Na+. Altogether, butyrate formation from CO occurs according to the following reaction: 10CO + 4H2O → 1 butyrate + H+ + 6CO2. This yields 3.5 mol ATP/mol butyrate and therefore considerably more ATP (Adenosine triphosphate) than does butyrate formation from H2 + CO2.
Prof. In Seop Chang said, “This result will help us understand the energy metabolism of other acetogen bacteria with similar characteristics of E. limosum KIST612. In addition, it would be possible by using metabolic engineering to create professionally designed uses for the butyratel production and waste gas converting acetogen bacteria.”