Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press release] Professor Youngjune Park"s research team identifies control factors for the carbon dioxide conversion process into high-value mineral carbonates
- 엘리스 리
- REG_DATE : 2016.12.26
- HIT : 1200
Professor Youngjune Park"s research team identifies control factors for the carbon dioxide conversion process into high-value mineral carbonates

(From left) M.S. student Dasol Choi, Ph.D. student Ribooga Chang, Professor Youngjune Park
□ Gwangju Institute of Science and Technology (GIST) Professor Youngjune Park"s research team has identified factors that control the crystal structure in the process of converting carbon dioxide, which is a greenhouse gas, permanently into high-value mineral carbonates *.
* Mineral Carbonate: A compound formed through the carbonation of carbon dioxide with alkaline earth metals, such as calcium and magnesium. Besides being able to store thermodynamically stable carbon dioxide permanently, the formed carbonate can be utilized as a reinforcing material for paper, polymers, chemicals, and construction material for buildings and other civil engineering projects.
The team identified three types of crystal structures that can be formed in the calcium carbonate (CaCO3) conversion process and suggested the key factors that determine the formation of those crystal structures and their correlations.
∘ Calcium carbonate forms a homogeneous polymorph of hexagonal calcite, vaterite, and aragonite. Depending on the purity and grain size of these crystals, calcium carbonate has various uses.
∘ Carbon dioxide can be converted to mineral carbonate by reacting with calcium or magnesium extracted from natural minerals or industrial by-products (slag, etc.) after the carbon capture process, such as absorption and adsorption. The crystal structure and purity of the carbonate are determined during the conversion process depending on such variables as the reaction temperature, pressure, pH concentration, the presence of impurities, and other factors.
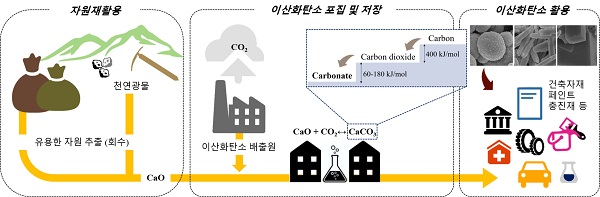
(Figure 1) Mineral carbonate process flowchart: Calcium or magnesium extracted from industrial by-products (slag, etc.) and natural minerals reacts with carbon dioxide, a greenhouse gas, and is converted into a more stable mineral carbonate by thermodynamic processes. Mineral carbonate can be used as a reinforcing material for construction and civil engineering projects.
∘ The researchers confirmed that these variables affect the formation process of the three crystal structures of calcium carbonate at different strengths. The reaction temperature is the most important control parameter for orthotropic aragonite, while ion concentration is the most important factor for the hexagonal calcite and baterite.
Ph.D. student Ribooga Chang said, "Certain calcium carbonate homogeneous bodies can be formed using a mixture of carbon dioxide and nitrogen gas, which can be stored without a separate carbon dioxide capture process."
□ GIST"s Earth Sciences and Environmental Engineering Professor Youngjune Park said, "We anticipate that the high cost related to applying CCS * technology will be solved to a large extent with our conversion process to high-value mineral carbonates."
* CCS (Carbon Capture & Storage): Carbon dioxide capture and storage. Technology that captures carbon dioxide from power plants and industrial processes, which are mass emission sources, and permanently preventing them from being released into the atmosphere.
□ This research was led by Professor Youngjune Park and conducted with support from the GIST Global University Project (GUP). Their paper authored by Ribooga Chang, Dasol Choi, Min Hee Kim, and Youngjune Park was published by ACS Sustainable Chemistry & Engineering, a well-known journal in the field of chemical engineering.