Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Professor Youngsoo Jun"s research team develops bioengineered yeast for targeted drug delivery to cancer cells
- 엘리스 리
- REG_DATE : 2016.01.12
- HIT : 852
Professor Youngsoo Jun"s research team develops bioengineered yeast
for targeted drug delivery to cancer cells
Saccharomyces cerevisiae genetically engineered to produce nanosized vacuoles displaying human epidermal growth factor receptor 2 (HER2)-specific affibody
for active targeting of cancer cells
Research performed in collaboration from KAIST with results published in PNAS
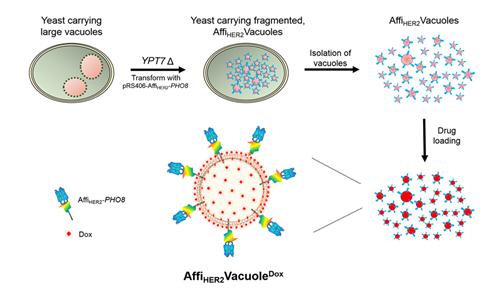
(Figure 1.) Generation and characterization of AffiHER2Vacuoles with a schematic illustration of fragmented vacuole generation and drug loading into anti-HER2 affibody-expressing vacuoles (AffiHER2VacuoleDox).
Synthetic nanomaterials are being studied for their effectiveness in delivering cancer drugs to malignant cells. Unfortunately, synthetic nanomaterials are hampered in their effectiveness as a viable cancer drug delivery vehicle because of several issues, such as their inherent toxicity as well as their inability to penetrate deeply into tumor tissue. Cancerous tumor tissues have formidable physiological barriers, such as a high interstitial pressure and a densely entangled extracellular matrix.
To overcome these challenges, a research team at GIST led by Professor Youngsoo Jun of the School of Life Sciences in collaboration with a research team at KAIST led by Professor Sangyong Jon of the Department of Biological Sciences, bioengineered nonhazardous and nonpathogenic Saccharomyces cerevisiae to produce nanosized vacuoles displaying human epidermal growth factor receptor 2 (HER2)-specific affibody for active targeting of cancer cells. These nanosized vacuoles efficiently loaded the anticancer drug doxorubicin (Dox) and were effectively endocytosed by cultured cancer cells. Their cancer-targeting ability, along with their unique endomembrane compositions, significantly enhanced drug penetration in multicellular cultures and improved drug distribution and penetration in a tumor xenograft while preventing tumor growth without eliciting immune responses.
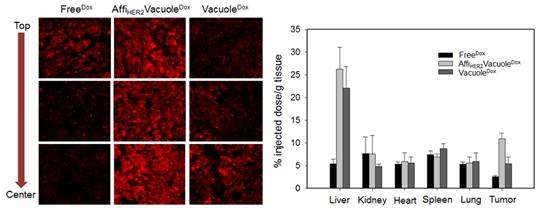
(Figure 2.) In vivo tumor targeting, biodistribution, and antitumor effects of AffiHER2VacuoleDox in NIH3T6.7 xenograft mice. Confocal images of tumor sections show the drug distribution in cancer cells after 6 h of treatment with AffiHER2VacuoleDox (1 mg/kg) compared with the controls. (Scale bars: 10 μm.) Tumor-specific distribution of AffiHER2VacuoleDox (1 mg/kg) analyzed 6 h after i.v. administration in NIH3T6.7 xenograft mice. Values are reported as the mean ± SD of triplicate samples.
Their research entitled "Bioengineered yeast-derived vacuoles with enhanced tissue-penetrating ability for targeted cancer therapy" was authored by Vipul Gujrati, Miriam Lee, Young-Joon Ko, Sangeun Lee, Daejin Kim, Hyungjun Kim, Sukmo Kang, Soyoung Lee,Jinjoo Kim, Hyungsu Jeon, Sun Chang Kim, Youngsoo Jun, and Sangyong Jon and published on December 29, 2015, in the online edition of PNAS. The research was supported by grants from the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Silver Health Bio Research Center at Gwangju Institute of Science and Technology.
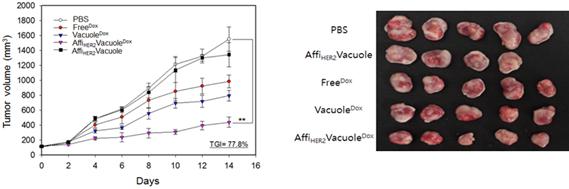
(Figure 3.) Tumor growth was monitored throughout the course of the treatment (Dox at a dose of 1 mg/kg administered every other day) in each treatment group. The percentage of tumor growth inhibition (TGI) caused by AffiHER2VacuoleDox was evaluated on the final day of treatment by comparison with PBS-treated controls; each value represents the mean ± SD (**P < 0.01 vs. control; n = 5 mice per group).

GIST Professor Youngsoo Jun KAIST Professor Sangyong Jon
GIST Professor Youngsoo Jun said, "This drug delivery approach was derived from the vacuole of a natural organism and provides inspiration and future directions for designing of an effective anticancer drug delivery system that could be considered in clinical applications to treat various cancers."