Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Research team led by Professor Jaeyoung Lee has developed a new catalytic method that is three times more efficient in converting carbon dioxide into biofuels than current methods
- 엘리스 리
- REG_DATE : 2015.10.30
- HIT : 945
Research team led by Professor Jaeyoung Lee has developed a new catalytic method that is three times more efficient in converting carbon dioxide
into biofuels than current methods
Research published in Angewandte Chemie International Edition represents a three-fold increase in carbon dioxide conversion rates over current methods and may offer new possibilities in controlling greenhouse gases while producing new biofuels
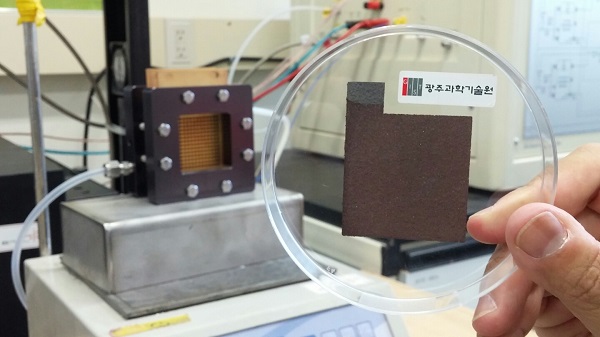
(Figure 1). Fabrication of Cu2O electrodes were made using an optimized electrode position method developed earlier by the same research team at GIST. Cu2O nanoparticles were deposited at 0.25 V (vs. RHE) in 0.5 M CuSO4 (> 99%) containing lactic acid (85~92%) and 5.0 M NaOH (> 97%).
GIST (Gwangju Institute of Science and Technology) researchers have developed a chloride (Cl)-induced bi-phasic cuprous oxide (Cu2O) and metallic copper (Cu) electrode (Cu2OCl) as an efficient catalyst for the formation of high-carbon organic molecules through carbon dioxide (CO2) conversion. This development may offer new possibilities in controlling greenhouse gases while producing new biofuels.
As industrialization releases more carbon dioxide emissions into the earth"s atmosphere and contributes to global warming, various carbon capture and sequestration (CCS) methods have been studied. However, CCS methods have a drawback in that they do not degrade or alter the basic composition of carbon dioxide over time, which creates the inherent risk of having massive quantities of C02 being re-released back into the environment through an accident or natural disaster. For that reason, carbon capture and utilization (CCU) methods have gained increased attention from researchers, and electrocatalytic conversion of CO2 is considered to be one of the most promising CCU technique. An important consideration for the feasibility of electrocatalytic conversion is having a high faraday efficiency, which is the electrical energy used to convert carbon dioxide into organic compounds.
GIST Professor Jaeyoung Lee (corresponding author) of the Ertl Center for Electrochemistry and Catalysis led a research team that was the first in the world to report the direct electrochemical production of various carbon 3 (C3) to carbon 4 (C4) products from CO2 with Faradaic efficiencies over 10% in a paper entitled "Electrocatalytic Production of C3-C4 Compounds by Conversion of CO2 on a Chloride-Induced Bi-Phasic Cu2O-Cu Catalyst" (the first author was Ph.D. candidate Seunghwa Lee) that was published online on October 16, 2015, in Angewandte Chemie International Edition. This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education.
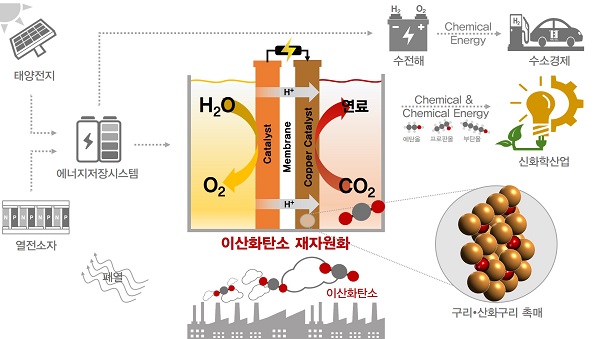
(Figure 2). Conceptualization of the electrochemical conversion cycle of carbon dioxide.
The research team demonstrated the efficient conversion of CO2 into multi-carbon fuels, particularly the C2, C3, and even C4 species chemicals, on an in situ formed Cl-induced bi-phasic Cu2O–Cu (Cu2OCl). Because of the chemoaffinity between chloride (Cl) and Cu, a uniquely shaped phase is formed by the application of a cathodic potential in the Cl-containing CO2 electrolytic system that is different from that formed when using a carbonate-containing CO2 electrolytic system. The use of a potassium chloride (KCl) electrolyte for the CO2 conversion on the Cu2O electrode leads to a higher suppression of the undesired hydrogen (H2) production and the improved preservation of the Cu2O phase.
The researchers also found that Cu2O was relatively stabilized and further transformed with the aid of Cl. The Cl-induced bi-phasic Cu2O–Cu showed remarkable catalytic ability toward multiple
C2–C4 species. In particular, higher carbon number products (≥ C3) were observed with highly significant Faradaic efficiencies of over 10%. Abundance of Cu1+ that can strongly bind and preserve the reaction intermediates for a longer time on the surface was demonstrated to be a key factor for the efficient synthesis of longer carbon chain during the CO2 conversion.
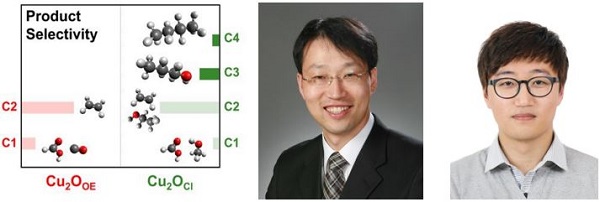
(Figure 3). Cl-induced bi-phasic Cu2O–Cu shows remarkable catalytic ability for CO2 conversion toward high-carbon number compounds. The oxidized Cu phase allows reaction intermediates to stay for a longer time on the surface, and consequently leads to formation of larger molecules. Professor Jaeyoung Lee (middle) and Ph.D. candidate Seunghwa Lee (right).
Professor Jaeyoung Lee said, "Developing a catalyst to convert carbon dioxide into organic compounds may contribute to solving environmental problems by controlling greenhouse gases while at the same time being able to produce more biofuels to alleviate the global energy crisis."