Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] A team led by Prof. Chang-Duk Jun identified how T-lymphocytes kill virus-infected cells
- 엘리스 리
- REG_DATE : 2015.05.20
- HIT : 1178
Identifying how T-lymphocytes kill virus-infected cells

Korean researchers have discovered the key molecular factor that kills virus-infected cells by stabilizing the attached Cytotoxic T Cell to the virus-infected cells. This discovery will open up new research possibilities in the medical world for defeating cancer as well as viruses, such as swine flu and bird flu.
Professor Chang-Duk Jun and a doctoral candidate Bo-Ra Na of the School of Life Sciences led the research with support from the Ministry of Science, ICT and Future Planning, National Research Foundation of Korea, and GIST Bioimaging Research Center. Their research paper entitled " TAGLN2 regulates T cell activation by stabilizing the actin cytoskeleton at the immunological synapse" was published on April 13, 2015, in The Journal of Cell Biology, which is one of the most prestigious journal in the field of cytology.
When the human body is infected with a virus, the virus can cause countless diseases from a simple cold to AIDS and other deadly plagues. Cytotoxic T Cell helps maintain a person"s health by detecting and destroying virus-infected cell.
* Cytotoxic T cell: T cells that secrete toxins that only targets and kills virus-infected cells, cancer cells, etc.
Virus-infected cells are killed by a lethal injection of toxins through a special structure called the immunological synapse. Until now, it has been impossible to keep immunological synapses stable, and, therefore, it was difficult to take advantage of immunological synapses to improve immunity or to treat cancer.
* Immunological synapse is a structure formed when T-cells are attached with the virus infected cells
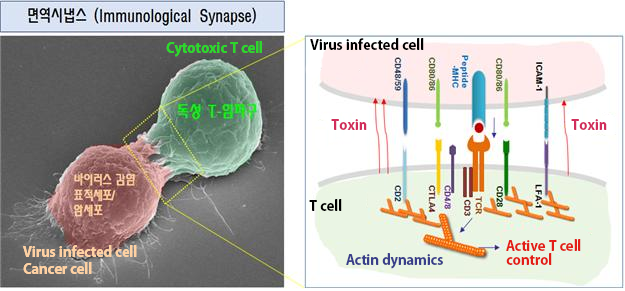
(Figure 1) Immunological Synapse structure and Cytotoxic T cell. Cytotoxic T cell forms a structure with the virus infected cell called immunological synapse. When observed closely by a microscope as in figure 1, MHC of the infected cell and TCR of the T cell are connected and the infected cell’s information is transferred to T cell. With various information (signals) received, actin-binding proteins are formed. By inducing the activity of LFA-1 (leukocyte function associated antigen-1) it increase cell adhesion and toxins are injected to the infected cells eventually destroying them.
The team discovered the TAGLN2 protein from the T cells that combines with actin to enhance the T cell"s fibrous structure, and this discovery was the first to find how TAGLN2 can maintain the stability of immunological synapses.
* Actin is the most abundant protein in cells. It is highly conserved and participates in more protein-protein interactions than any known protein. These properties, along with its ability to transition between monomeric (G-actin) and filamentous (F-actin) states under the control of nucleotide hydrolysis, ions, and a large number of actin-binding proteins, make actin a critical player in many cellular functions, ranging from cell motility and the maintenance of cell shape and polarity to the regulation of transcription
In animal testing, the genes that create TGALN2 protein was removed in a mouse and Cytotoxic T cells could not kill the virus-infected cells, but Cytotoxic T cells bound strongly to the virus-infected cells and destroyed them in a mouse with the TGALN2 protein.
The team is currently developing a peptide compound that modulate the function of the TAGLN2 protein. This substance could help increase the body’s resistance to viruses and may also help create Cytotoxic T cells within cancer cells. The researchers are also hopeful that this discovery could also lead to new treatments for patients of organ transplants to avoid organ rejection.
Prof. Jun said, “It is important to acknowledge that Korean researchers discovered the source material for the basis of this biological immune response. This will help us develop an advanced health care industry within Korea that is based on our own domestic core technologies without having to be dependent on foreign technologies.”