Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
Prof. Jang-Soo Chun"s Team Identifies Regulator of Rheumatoid Arthritis
- 정명식
- REG_DATE : 2014.07.11
- HIT : 702
□ A Korean research team revealed that the HIF-2α (hypoxia-inducible factor-2α) is a key transcription factor regulating rheumatoid arthritis (RA). Their achievement is expected to provide a therapeutic target for the development of RA cures. The results were published in the June 10th issue of PLOS Biology (Title: Hypoxia-inducible factor-2α is an essential catabolic regulator of inflammatory rheumatoid arthritis). The research was conducted by a team jointly led by Prof. Jang-Soo Chun of GIST (School of Life Sciences) and Prof. Je-Hwang Ryu of Chonnam National University (School of Dentistry), and was supported by the National Core Research Center program of the National Research Foundation (NRF) and the Ministry of Science, ICT, and Future Planning and by the General Researcher program of NRF. □ Characterized by chronic inflammation in joint tissues, RA is known as a disease in which immune cells abnormally activated by autoimmunity destroy cartilages, ligaments, and bones in joints. However, the exact causes of RA remain unknown, and its treatment relies mainly on alleviating symptoms instead of treating fundamental causes. □ The research team found out that HIF-2α, whose expression is substantially increased in joint tissues of RA patients or animals, leads to the development and progression of RA by directly regulating FLS (fibroblast-like synoviocytes). Therefore, HIF-2α is expected to serve as a new target for the development of RA cures. □ When HIF-2α was injected into the knee joints of mice, RA-like symptoms appeared without using any RA-inducing agents. Conversely, in the knock-out mice lacking HIF-2α, when induced by RA-inducing agents, the RA symptoms were less severe. The reason is believed to be that HIF-2α is involved in the activation of osteoclasts that destroy bones and the expression of various proteins that help destroy joints. □ Biological formulations that control inflammation mediators can lead to a more effective RA treatment by removing the fundamental causes eliciting the disease, instead of just relieving the symptoms. “The results will lead to the development of a more fundamental and more effective treatment of RA by suppressing HIF-2α, which was discovered as a RA-causing protein,” said Professor Jang-Soo Chun.

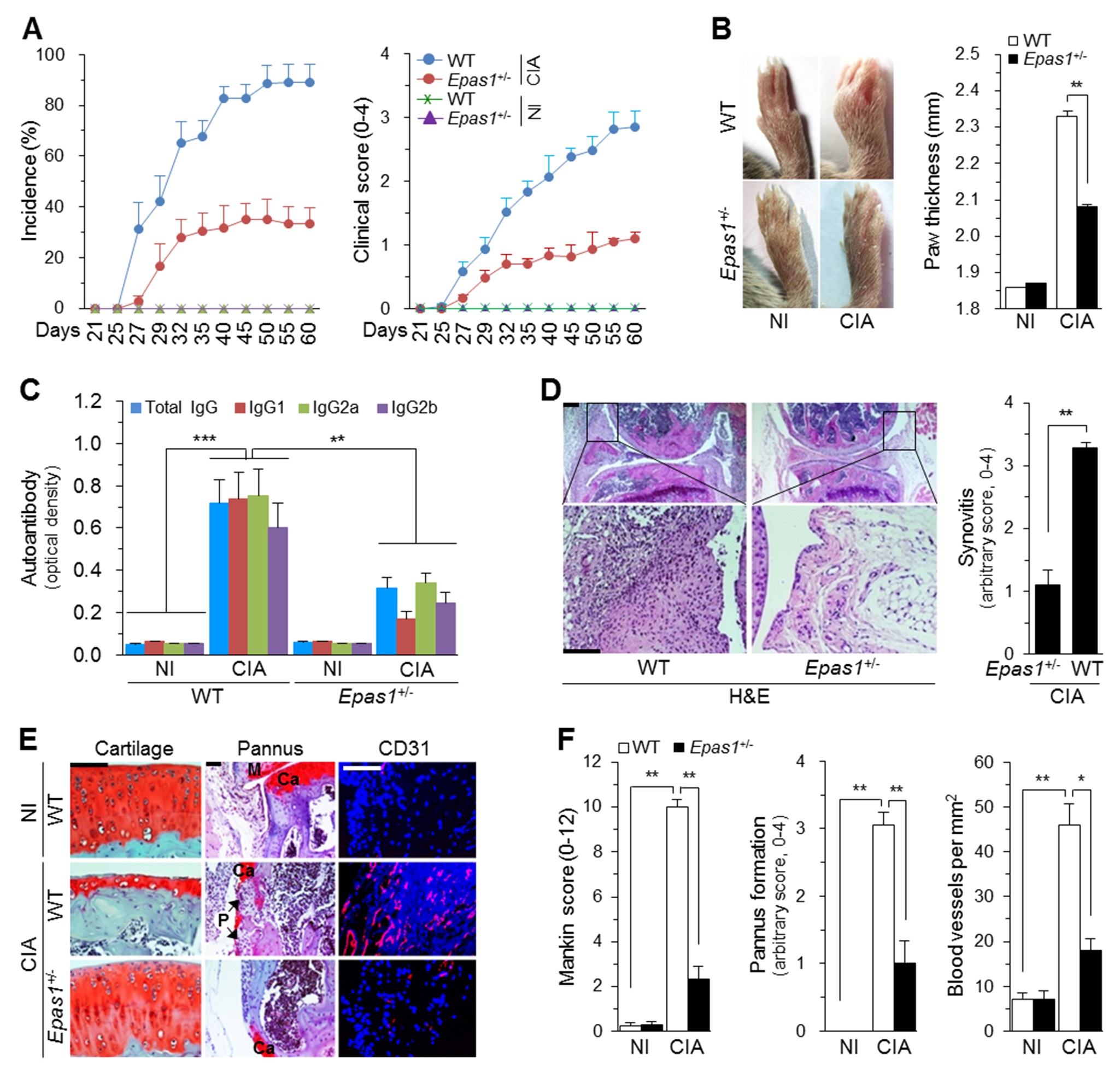
(A) Incidence and severity of CIA in WT and Epas1+/− DBA/1J mice without (NI) and with CIA (n = 20 mice per group). (B) Typical paw images on day 60 after the first immunization and ankle thickness measured with a digital thickness caliper (n = 20 mice per group). (C) Type II collagen-specific autoantibody production under NI and CIA conditions in the sera of WT and Epas1+/− DBA/1J mice (n = 12). (D) H&E staining and scoring of synovial inflammation (n = 10). (E) Representative images of safranin-O staining of articular cartilage, safranin-O/hematoxylin staining of the pannus, and immunofluorescence microscopy of CD31 in knee joints of WT and Epas1+/− DBA/1J mice without (NI) and with CIA. (F) Quantification of results in (E). Mankin score (n = 12), pannus formation (n = 10), and number of blood vessels in the synovium (n = 10). Ca, cartilage; P, pannus; M, meniscus. Values are means ± SEM (*p<0.01, **p<0.001, ***p<0.0005). Scale bar, 50 µm.