Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press Release] Professor KwangSup Eom develops Li-ion battery that doubles the distance of electric vehicles
- 엘리스 리
- REG_DATE : 2017.01.10
- HIT : 1189
Professor KwangSup Eom develops Li-ion battery
that doubles the distance of electric vehicles
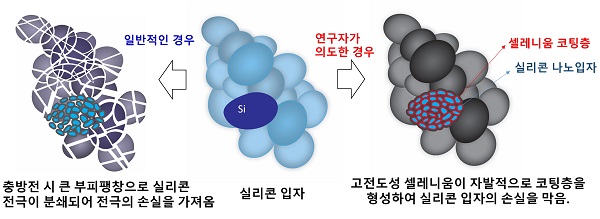
[Figure 1] A simplified schematic diagram of ideas for improving the stability of the new batteries. In general, a silicon-sulfur battery breaks down into nano-sized silicon anode materials due to volume expansion during charging and discharging, and a silicon anode is contaminated by a polysulfide synthesized from a sulfur electrode. The key idea of this study is to continuously maintain the conductivity of silicon by coating selenium-lithium compound on silicon anode by adding selenium with high electrical and ionic conductivity to the sulfur electrode to prevent performance decrease. Through this method, mutual positive effect (synergy effect), which improves the stability of both the silicon anode and the sulfur anode, was obtained.
□ A joint research team including GIST Professor KwangSup Eom from the School of Material Science and Engineering and Dr. Jung Tae Lee (joint first author) of MIT Electronics Engineering Research Institute has developed a new lithium ion battery with four times the capacity and more than double the lifetime of currently used lithium ion batteries.
∘ When the battery is commercialized, the maximum mileage of an electric car will increase to about 300 km at the time with one charge, the lifetime of a cell phone battery will be doubled, and the weight will be reduced to half.
Currently, graphite (cathode) and lithium metal oxide (anode) are used as electrode materials for commercial lithium ion batteries. Both materials have a relatively low energy storage capacity and are approaching the theoretical capacity * with current technology, and are facing the limit of increasing the electric storage capacity of electric vehicles, which are mainly used for short-distance driving.
* Theoretical capacity: The inherent maximum lithium storage capacity (= charge storage capacity) of an electrode material for a lithium ion battery. Experimentally, it cannot be higher. For example, in the case of graphite, one lithium ion is stored per 6 carbon atoms, which is calculated as 374 mAh / g.
∘ To increase the distance traveled by electric vehicles, it is possible to install a large amount of batteries. However, since the weight of the vehicle body increases and the fuel consumption of the automobile decreases, there is a limit to increase the mileage by adding the battery only. Therefore, new batteries must be developed using new electrode materials that have a large storage capacity per weight and volume.
The team developed a new lithium-ion battery with more than twice the capacity (more than twice the energy density) and lifetime of more than twice the capacity per weight of current lithium-ion battery using lithium / silicon (cathode) and sulfur / selenium.
∘ The team looked at "silicon cathode" and "sulfur anode" as new electrode material for lithium ion batteries and added selenium (Se) * to the sulfur anode to form a "lithium / silicon cathode" - "sulfur / selenium anode" battery. As a result, before the sulfur was dissolved, the cellulose was preferentially dissolved and was preferentially included in the solid electrolyte interface (SEI) layer of the silicon electrode, and the performance was not reduced while it served as a protective film for the silicon cathode.
* (Supplementary explanation) When sulfur is dissolved in the anode, diffusion through the electrolyte will flow into the surface oxide layer of the anode as well as on the anode, resulting in a rapid drop in the ion and electrical conductivity of the silicon cathode. To solve this problem, selenium, which has ion and electric conductivity of several thousands to several hundred thousand times higher than sulfur, is added as a substance that positively affects the anode oxide layer.
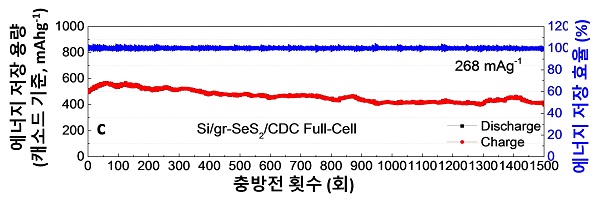
[Figure 2] Development graph showing the actual measured energy storage capacity per charge / discharge cycle of a new battery (silicon / graphene cathode - sulfur / selenium anode). The red data in the graph means the energy storage capacity (mAh / g) recorded per charge and discharge, and the blue data means the charge and discharge energy storage efficiency, which is about 99.99%.
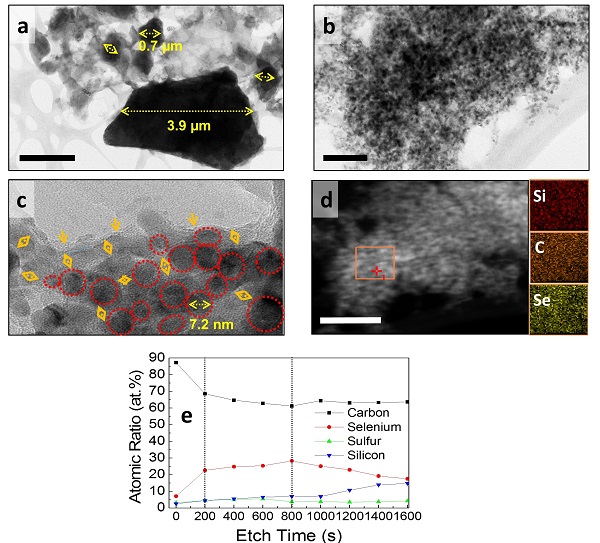
[Figure 3] Electron microscope photographs (a to d) of the electrode material before and after 1000 times of charging and discharging of the new battery and the chemical composition data analysis. The selenium component is uniformly coated on the inside of the nano sized silicon electrode.
The developed lithium-ion battery has a storage capacity of about 500mAh / g, which is about four times larger than the currently commercialized lithium-ion battery (100-150 mAh / g) and about twice the energy density * respectively.
∘ That is, when the new battery is used for an electric car, the energy storage density per charge / discharge is more than twice that per weight, so if you use the same weight battery, the distance (about 300 km) can be increased.
In particular, the researchers focused on improving the stability of the battery, resulting in a performance reduction of only 19% during 1500 charge / discharge cycles. This means that even if used once a day for about 4 years (365 times × 4 = 1460 times), the battery performance is maintained over 80% and can be used without replacement. (Batteries for electric vehicles should be able to maintain more than 80% of their performance during operation)
∘ In addition, even if the developed battery is used in a mobile phone, the user does not notice a significant decrease in performance (less than 20% in performance) for four years. When designing the same capacity as the current cell phone battery, the battery weight decreases by about half.

GIST Professor KwangSup Eom and MIT Dr. Jung Tae Lee
□ GIST Professor KwangSup Eom said, "Commercialization of the developed battery can dramatically increase the mileage of an electric car by 150km with a single charge. Additional types and amounts of additives are expected to increase capacity by 1.5 times and increase life span by more than two times."
□ This study, led by GIST Professor KwangSup Eom and Dr. Jeong-Tae of MIT, under the auspices of the Professor Gleb Yushin and Professor Thomas Fuller of the Georgia Institute of Technology was published online on January 5, 2017, in Nature Communications.