Media Center
A multimedia mosaic of moments at GIST
GIST Excellence
[Press release] Professor Myung-Han Yoon leads a research team to develop nanowire-forest to produce hydrogen from water molecules
- 엘리스 리
- REG_DATE : 2016.10.17
- HIT : 877
Professor Myung-Han Yoon leads a research team to develop
nanowire-forest to produce hydrogen from water molecules
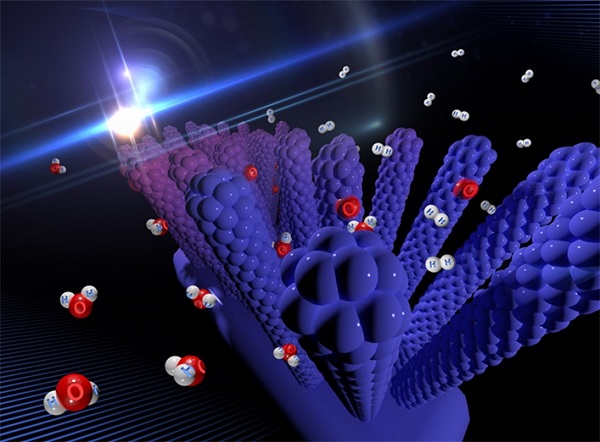
Image 1: Professor Myung-Han Yoon"s paper "Hydrogen production based on a photoactivated
nanowire-forest" was selected as the cover article for the Journal of Materials Chemistry A.
Hydrogen, which is the most abundant element in the universe, is considered to be a promising energy source for the future because of its inherent renewability and environmental-friendliness. However, a hydrogen-based energy economy would require a greater increase in the production of hydrogen, and this poses a problem because the current industrial method usually requires high-temperate steam between 700°C–1,000°C to produce hydrogen from a methane source, which creates greenhouse gases and diminishes its renewability.
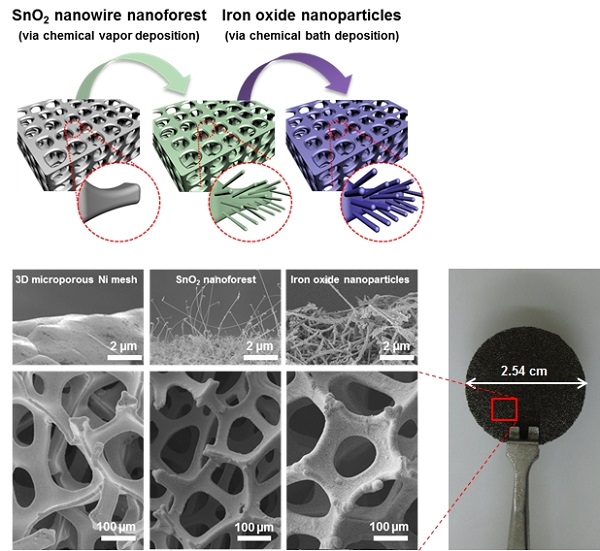
Image 2: Schematic of the two-step dip-coating method for iron oxide nanoparticle
film formation on 3D Ni foam and SnO2 nanowires grown on Ni foam scaffolds.
To address this problem, Professor Mung-Han Yoon of the School of Materials Science and Engineering at the Gwangju Institute of Science and Engineering (GIST) has led a research team to develop a low-temperature thermochemical method to produce hydrogen from water molecules that uses hierarchically assembled iron oxide nanoarchitectures. The hierarchically arranged catalyst was prepared by loading iron oxide nanoparticles onto a tin oxide (SnO2) nanowire forest on a microporous nickel scaffold.
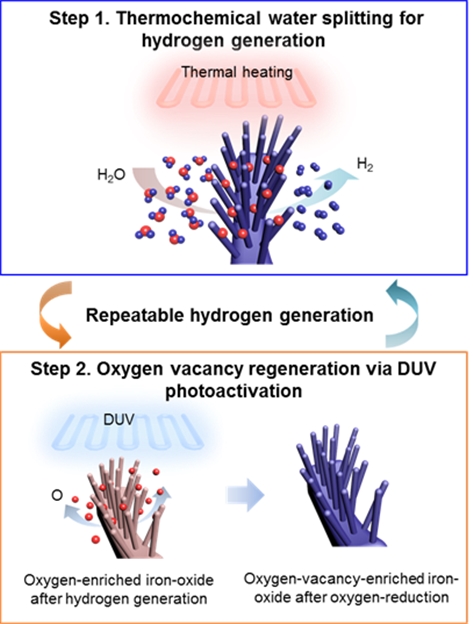
Image 3: Oxygen reduction process on oxygen-enriched iron oxide nanoforests
using DUV exposure (254/185 nm) and low-temperature annealing (200 °C).
The research team was able use this method of produce hydrogen at temperatures of 400–800 °C, with a high specific gas-forming rate as high as ∼25 000 μmol per g per 250 minute cycle. More remarkably, deep-ultraviolet photoactivation enabled low-temperature (200 °C) catalyst regeneration and thereby allowed multiple cycles of hydrogen production without any significant coalescence of the oxide nanoparticles nor substantial loss of the water-splitting efficiency.

Image 4 (from left): Professor Myung-Han Yoon, Ph.D. student Seyeong Lee, and Dr. Zahid Hanif
Their paper "Hydrogen production based on a photoactivated nanowire-forest" was authored by Seyeong Lee, Zahid Hanif, Keumyoung Seo, Taekyung Lim, Hye-Min Shin, Sungjun Park, Su Hwan Kim, Sang Kyu Kwak, Sukwon Hong, Myung-Han Yoon, and Sanghyun Ju and was published the Journal of Materials Chemistry A on September 14, 2016.
Professor Myung-Han Yoon said, "Our research utilizes nano-material technology with photoactivation to produce hydrogen from water molecules, and this process will have a variety of applications in different research fields."